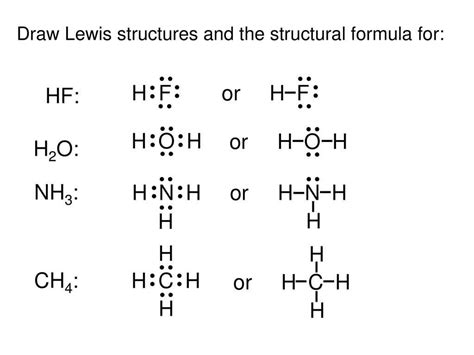
nh3 electron dot structure|Iba pa : Baguio This chemistry video tutorial explains how to draw the lewis structure of NH3 also known as Ammonia.How To Draw Lewis Structures: https://www.you.
Come & Visit us on SFUC Diagnostics Mexico and consult to our doctors ☺️ Dr. Anne M. Galvan (Ob-Gyne) Monday & Friday (1:30pm-3:00pm) See you! ☺️
PH0 · nh3 structure geometry
PH1 · nh3 lewis structure chem 101
PH2 · lewis structure for nh3
PH3 · lewis dot structure of nh3
PH4 · lewis dot diagram maker
PH5 · lewis dot diagram for ammonia
PH6 · electron geometry of nh3
PH7 · electron dot diagram for nitrogen
PH8 · Iba pa
STEAM CHARTS An ongoing analysis of Steam's concurrent players. Realm Royale Reforged. Store | Hub. 311 playing . 325 24-hour peak 104557 all-time peak Compare with others. Month Avg. Players Gain % Gain Peak Players; Last 30 Days: 246.60 +3.8 +1.55%: 441: August 2024
nh3 electron dot structure*******In the NH3 lewis dot structure, there are Four atoms present, one N and three H atoms. The valence electrons for N are 5 and for three H atoms are 3. So, the .
Learn how to draw the Lewis structure of ammonia (NH3) with 8 valence electrons and sp3 hybridization. Find out the molecular .
NH 3 (Ammonia) is a commonly tested Lewis structure. It's not particularly difficult but is an important structure. In the NH 3 Lewis structure (and all structures), hydrogen goes .
nh3 electron dot structure Iba paTo begin drawing the NH3 Lewis structure, start by counting the total number of valence electrons. Valence electrons are the outermost electrons of an atom and are involved in .Ammonia (NH 3) is a commonly tested Lewis structure due to it's widespread use in agriculture as a fertilizer. It also is a good example of a molecule with a trigonal prymidal .
This chemistry video tutorial explains how to draw the lewis structure of NH3 also known as Ammonia.How To Draw Lewis Structures: https://www.you.Ammonia (NH 3) Lewis Structure | Steps of Drawing. In the lewis structure of ammonia (NH 3), there are three N-H bonds and one lone pair on nitrogen atom. Lewis structure of NH . NH3 Lewis Structure: NH3 (Ammonia) has a trigonal pyramidal structure: central N atom with 5 valence electrons forms 3 N-H single bonds, using 3H atoms (1 .
This chemistry video tutorial explains how to draw the lewis structure of NH3 also known as Ammonia.How To Draw Lewis Structures: https://www.you.
So we're going to a lot more examples for drawing dot structures in the next several videos, and see how drawing dot structures allows you to predict the shapes of different molecules. Learn for free about math, art, computer programming, economics, physics, chemistry, biology, medicine, finance, history, and more.Step-by-Step Guide to Drawing NH3 Lewis Structure. 1. Count the Total Number of Valence Electrons. To begin drawing the NH3 Lewis structure, start by counting the total number of valence electrons. Valence electrons are the outermost electrons of an atom and are involved in bonding. For NH3, nitrogen (N) is in Group 5A (Group 15), so it has .
Transcript: OK, this is Dr. B. We're going to do the Lewis structure for NH3: ammonia or Nitrogen trihydride. On the periodic table, Nitrogen is in group 5 or 15 so it has 5 valence electrons, and then Hydrogen is in group 1. It has one valence electron, but we have 3 Hydrogens, so let's mutiply that by 3.Iba pa Follow these simple steps to draw Lewis dot structures: Draw the atoms on paper and put dots around them to represent valence electrons of the atom. Be sure to have the correct number of electrons. If the species is an ion, add or subtract electrons corresponding to the charge of the ion. Add an electron for every negative (-) charge, .
We can illustrate the formation of a water molecule from two hydrogen atoms and an oxygen atom using Lewis dot symbols: . (NH3) whose Lewis electron structure is as follows: A neutral nitrogen atom has five valence electrons (it is in group 15). From its Lewis electron structure, the nitrogen atom in ammonia has one lone pair and shares three . The central atom in a structure often violates the octet rule. Electron-deficient atoms are rare, but expanded octets are fairly common with elements in the 3 rd row and beyond. In BF 3, the central atom only has 6 electrons around it. We say that the boron atom is electron deficient. In BrF 3, the central atom has 10 electrons around it.
Hydrogen (H) is in Group 1, having 1 valence electron. Since NH3 has one nitrogen atom and three hydrogen atoms, the total number of valence electrons is 5 + (3 * 1) = 8. Sketch the Skeleton Structure: Place the nitrogen atom in the center because hydrogen cannot be a central atom. Draw single bonds between the nitrogen atom and .
Ammonia or NH3 has a total of 8 valence electrons. NH3 Lewis Structure. The Lewis structure of a molecule helps understand the electron geometry, molecular geometry, polarity, and other such properties with ease. It is a pictorial representation of the arrangement of valence electrons around the individual atoms in the molecule.
In this video we look at the electron geometry for Ammonia (NH3). Because the ammonia molecule has four electron domains (the three electron clouds around ea.Ammonia (NH 3) is a commonly tested Lewis structure due to it's widespread use in agriculture as a fertilizer. It also is a good example of a molecule with a trigonal prymidal molecular geometry. There are 8 valence electrons available for the Lewis structure for NH 3. Video: Drawing the Lewis Structure for NH3.
Nitrogen has 5 valence electrons, and hydrogen has 1 valence electron. They interact through covalent bonding to form ammonia (NH3). 3 hydrogen atoms share one electron with 1 atom of Nitrogen such that both have stable electronic configurations (octet for nitrogen and duplet for hydrogen). The Lewis electron dot structure for a molecule of . Step 3: Connect each atoms by putting an electron pair between them. Now in the NH3 molecule, you have to put the electron pairs between the nitrogen atom (N) and hydrogen atoms (H). This indicates that the nitrogen (N) and hydrogen (H) are chemically bonded with each other in a NH3 molecule. Step 4: Make the outer atoms stable.
NH3 is a neutral atom, N has 3- charge and H +1, in NH4 the N forms a dative covalent bond with the H (since H will probs lose it´s electron, +1), nay, the overall charge will be+1 . The method your teacher used is best when you know the electron-dot structure of the molecule ( e.g, H-O-SO₂-OH).A video explanation of how to draw the Lewis Dot Structure for Ammonia, along with information about the compound including Formal Charges, Polarity, Hybrid . The total valence electrons available for drawing the Ammonia (NH3) Lewis structure is 8. The molecular geometry or shape of NH 3 is a Trigonal pyramidal. The electron geometry of NH 3 is Tetrahedral. Because 1 lone pair and three bond pairs around the Nitrogen (N) central atom are arranged tetrahedrally.
Toppr: Better learning for better results . /ask/404/

Explanation: The Lewis structure of ammonia, N H 3, would be three hydrogen atoms bonded to a nitrogen atom in the middle, with a lone pair of electrons on top of the atom. This is the reason why ammonia acts as a Lewis base, as it can donate those electrons. Answer link.
That means you will need 6,300,000 units in order to maximize the number of slots in the exosuit. Upgrading your inventory is hugely important in No Man's Sky. It allows you to carry more, which .
nh3 electron dot structure|Iba pa